Research
My research program focuses on the interplay between parasites and host phenotype, with an emphasis on how phenotype-manipulating parasites alter host behavior. I’m interested in the mechanisms through which parasites manipulate host phenotype, as well as the ecological and evolutionary implications of manipulation.
My study systems California killifish (Fundulus parvipinnis) and their brain-infecting trematode parasite, Euhaplorchis californiensis (EUHA), and the crypt gall wasp (Bassettia pallida) and their parasitoid the crypt-keeper wasp (Euderus set). More details about my main projects can be found below.
MANIPULATION OF THE CRYPT GALL WASP (BASSETTIA PALLIDA) BY THE CRYPT KEEPER WASP (EUDERUS SET)
MANIPULATION OF THE CRYPT GALL WASP (BASSETTIA PALLIDA) BY THE CRYPT KEEPER WASP (EUDERUS SET)
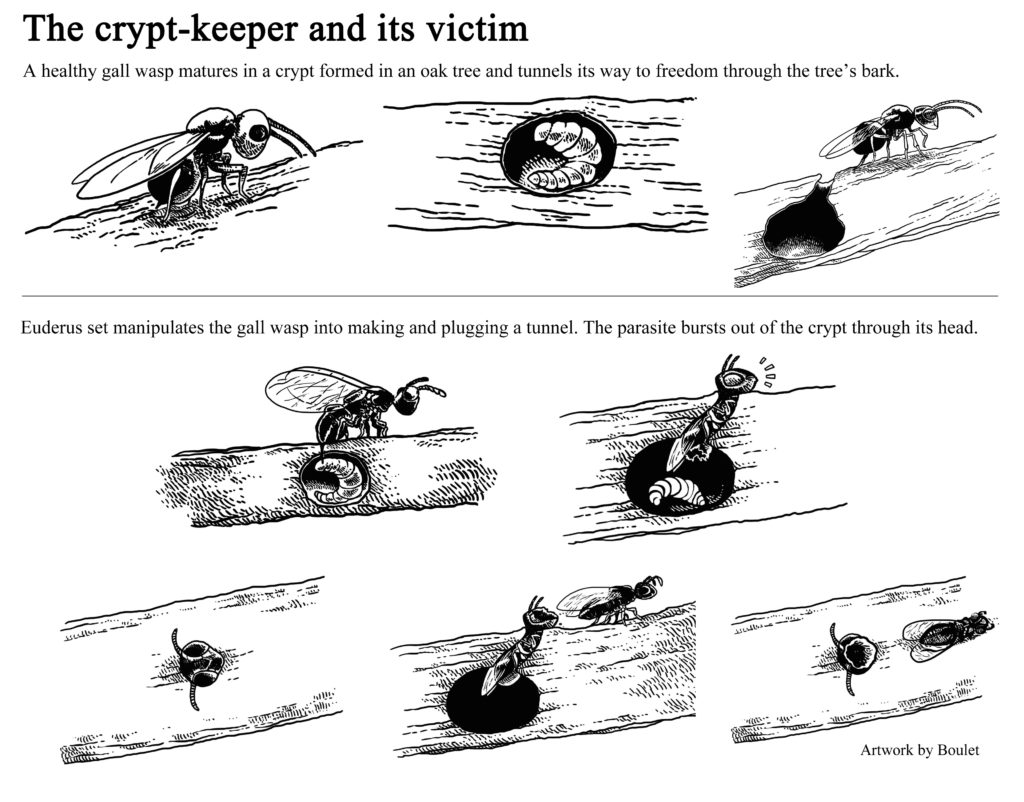
My collaborators and I discovered a parasitoid wasp that manipulates the behavior of its gall wasp host. See the manuscripts, video, press coverage, and artwork (by the amazing Boulet) below for more information about this study system.
 Previous publications in this system:
Previous publications in this system:
Description of Euderus set, the crypt-keeper wasp.
Study documenting that Euderus set is more likely to die trapped in crypts when they have to chew through bark to escape, which suggests that Euderus set manipulates Bassettia pallida to create an emergence hole because Euderus set would have difficulty creating this emergence hole on its own.
Popular press coverage of this research:
MANIPULATION OF CALIFORNIA KILLIFISH (FUNDULUS PARVIPINIS) BY THE BRAIN-INFECTING TREMATODE EUHAPLORCHIS CALIFORNIENSIS
MANIPULATION OF CALIFORNIA KILLIFISH (FUNDULUS PARVIPINIS) BY THE BRAIN-INFECTING TREMATODE EUHAPLORCHIS CALIFORNIENSIS
California killifish (Fundulus parvpinnis) infected by the trematode Euhaplorchis californiensis exhibit conspicuous behaviors that appear to draw the attention of the parasites’ definitive host (predatory birds) (Lafferty & Morris 1996).
 Current projects in this system include exploring 1) the mechanisms through which Euhaplorchis californiensis manipulates killifish behavior, 2) the extent to which infection influences killifish “personality traits” including social behavior, antipredator behavior, and activity levels, and 3) how the expression of manipulated behaviors change across the lifetime of the killifish as they continuously acquire infections.
Current projects in this system include exploring 1) the mechanisms through which Euhaplorchis californiensis manipulates killifish behavior, 2) the extent to which infection influences killifish “personality traits” including social behavior, antipredator behavior, and activity levels, and 3) how the expression of manipulated behaviors change across the lifetime of the killifish as they continuously acquire infections.
Previous publications in this system:
Euhaplorchis californiensis cercariae show no evidence of crowding, and may even benefit from the presence of conspecifics.
Low dose infections with Euhaplorchis californiensis do not alter host behavioral type.
The interaction between handling stress and Euhaplorchis californiensis density is associated with cortisol release rates in California killifish.
Euhaplorchis californiensis cercariae are positively phototaxic and negatively geotactic.
ALTERNATING REPRODUCTIVE TACTICS IN SMALLMOUTH BASS (MICROPTERUS DOLOMIEU)
ALTERNATING REPRODUCTIVE TACTICS IN SMALLMOUTH BASS (MICROPTERUS DOLOMIEU)
In many animals, reproducing males adopt distinct approaches to reproduction – with males that adopt different approaches often looking and acting quite different from one another. Hooknose and jack males in salmon populations are well-known examples, where the hooknose males are large, aggressive, and attract females while the jack males are small, inconspicuous, and sneak into nests to deposit sperm while females are mating with hooknose males.
In systems like this, the males are said to exhibit “alternative reproductive tactics” or ARTs. ARTS are often not inherited genetically, and instead depend on the conditions a male experiences as he grows up. Smallmouth bass (Micropterus dolomieu) exhibit alternative reproductive tactics, where some males breed for the first time at age 3 while others wait to breed until a later year. We collected data on all breeding males in a lake in northern Wisconsin every year for a decade, and are using data on these males and on the conditions in the lake each year to explore the reasons why males ending up adopting a particular tactic. This project was recently funded by the National Science Foundation (IOS-1755421 “Collaborative Research: Expression and dynamics of reproductive tactics in a wild population of smallmouth bass ” awarded to Dan Wiegmann, Scott Egan, and I).
