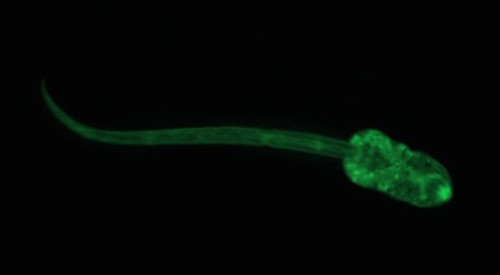
Recently I tried out a new dye on the cercariae that I’m studying. Cercariae are the free-swimming stage of trematode parasites that search for the next host in their life cycle. For my research, I’m studying the trematode Euhaplorchis californiensis (EUHA), and their cercariae are on the look-out for California killifish (Fundulus parvipinnis). I would like to be able to infect wild-caught fish in the lab, and be able to differentiate between the parasites that the fish became infected with while they were living in the wild from those parasites that they acquire when I infect them in the lab. To differentiate between lab and field infections I was hoping to mark cercariae from lab infections with a fatty-acid analogue dye called BODIPY FL C12. This dye binds to lipids in cell membranes, and it “glows” when the dye is excited by photons. Here is what EUHA looks like after it has been stained with the dye:
Look at its little eyespots. What a cutie.
The parasites were very active, which suggests that the dye wasn’t harming them in any way. Unfortunately, the dye wears off very quickly in the presence of light. I was quite surprised at how quickly the dye stopped fluorescing, and after 5 minutes in a completely dark room (except for the light from the microscope) it was nearly impossible to see the cercariae. This is a big problem for me, because California killifish have somewhat transparent skulls, so I imagine that the dye would wear off pretty quickly. Soooo, I probably won’t get a chance to use this technique for my experiment, but it sure was fun making my darling little brain parasites glow!

What about using something like Biolume? I don’t know how available it is, though.
I haven’t checked out Biolume yet. I’ll look into it! Thanks for the suggestion.
Have you tried using red light instead of white, maybe it wouldn’t degrade as fast? Also, if you are sacrificing the killfish (as oppose to wanting them to live) have you thought of using a pcr or aflp type technique? If you had a somewhat homogenous strain of lab parasites you could potentially use genetics to tell them apart from wild type cercariae. Can you extract DNA of the cercariae?
Hey, Jodell.
Thanks for the suggestion! I will eventually want to euthanize the killifish and it would be easy to get a homogenous lab strain of the parasite (they reproduce asexually in their snail hosts and they reproduce continually, so as long as I keep track of snails I can keep track of genotypes). While I haven’t extracted DNA from cercariae (I’m relatively new to molecular techniques), I know that it can be done. Unfortunately, I don’t have money for the preliminary work necessary to run PCR of aflp on my parasite, nor do I have money to process the 100+ fish that I’ll be running through experiments. Hopefully some of the grants I wrote go through and then perhaps this could be an option!
Hmm, why would red light degrade slower than white (although I was actually using a green dye)?
I think it might be because red light is at a higher wavelength, so it’s less energetic. The red light might not cause the dye to fluoresce though…
Have you tried BODIPY 558/568 C12 (a red dye)? I found an article where they use both BODIPY 558/568 C12 and FL C12 to effectively stain trematoads without effecting the organisms (“The use of fluorescent fatty acid analogs as labels in trematode experimental infections” by Keeney, et al.).
Good luck in your research!
We were following the methods from the Keeney et al. paper and were using BODIPY FL C12. Thanks! 🙂
If there’s any way to genetically engineer them, transforming with GFP or some other fluorescent protein would work, and be permanent.
There might be, but that would take a lot more time and money that I have at the moment. I’m working on lab breeding killifish now so I don’t have to be worry about differentiating between lab and field infections. While it would be a lot more fun to genetically engineer them, this will hopefully cost less and take less time. Thanks for the comment!
Hey!
That sounds like a lot of my PhD…all these awesome experiments and not enough money! I was worried about the red light not making them flouresce too. I think it is due to the wavelength. We use it on our flies when we are trying to not mess up their circadian rhythms, thought’d it be worth a shot for you. Have you ever tried applying for a Sigma Xi grant? It’s a bit hard to get if your mentor isn’t in Sigma Xi, but they are super easy to apply to, deadlines are twice a year and give up to 1000 bucks. I used it to get get some real time rt PCR supplies when my lab was broke so I could do some gene expression. They like eco/evo stuff too. Good luck!
Hey!
I have applied for Sigma Xi grants, and a bunch of others! 🙂 I’m actually waiting to hear back on a handful of grants, though most of that money is slated for buying hormone EIA kits. Thanks! 🙂
..and good luck with your PhD! 🙂
I hope you get your money! I actually finished a couple of years back. I’m about to start a new lab manager job at Vanderbilt working on virus. Should be really exciting. 🙂
Thanks! Have fun with the viruses!! I’m doing my best to learn about viruses during my down time by listening to This Week in Virology. Almost makes me wish I studied viruses!
“my darling little brain parasites”
There’s a phrase you don’t hear too often.
I agree, though. It is cuddly.
Hello,
I doubt wavelength is the bigger issue. The room light will not really be as important as the lamp itself. How long were you looking at the cercariae until bleaching occured? During that time the lamps was always on? Try and look for a microscope that has neutral density filters to cut down on the light intensity and has a good, sensitive camera. As a minor note, all dyes are sensitive to pH and oxygen so maybe your little bug’s burst of activity is causing faster bleaching.
Have you looked at the FM membrane dyes? They are not necessarily more stable, but maybe they less sensitive to your particular needs. Invitrogen are fairly open to giving them a call and asking for a sample around here, so maybe you can try?
http://probes.invitrogen.com/media/pis/mp34653.pdf
Invitrogen also have a wide range of dyes for your purpose, you can look here if you want to explore others:
http://www.invitrogen.com/site/us/en/home/References/Molecular-Probes-The-Handbook/Fluorescent-Tracers-of-Cell-Morphology-and-Fluid-Flow/Tracers-for-Membrane-Labeling.html#head9
Thanks for the comment! This was very helpful!
Good to know! If you have microscopy questions feel free to email me (I’m a fluorescence microscopy specialist in Lisbon).
Will you update about your project? I’m curious now!
Howdy would you mind sharing which blog platform you’re using?
I’m planning to start my own blog in the near future but I’m having a difficult time choosing between BlogEngine/Wordpress/B2evolution and
Drupal. The reason I ask is because your layout
seems different then most blogs and I’m looking for something completely unique.
P.S My apologies for getting off-topic but I had to ask!
My friend set it all up, but I’m using the Faculty theme from Theme Forest.