I recently received a grant from the Animal Behavior Society to buy enzyme immunoassay kits. These kits will be used to measure how cortisol (a “stress hormone”) concentrations influence the ability of trematode parasites to infect California killifish (i.e., are killifish with higher cortisol levels more likely to become infected by their trematode parasites?), and to measure how cortisol concentrations change after infection. Why I’m asking these questions is a good story for another day. Today I want to tell you about how the kits I’ll be purchasing work, because they’re AWESOME:
Below is a picture of a finished plate. In this case, the second and third columns were used as standards. If you look carefully, you can see that they start very faint, and get more bright as you move towards the bottom. This is because more of the kit hormone (bound to the tracer) has been added to each well as you progress down the plate.
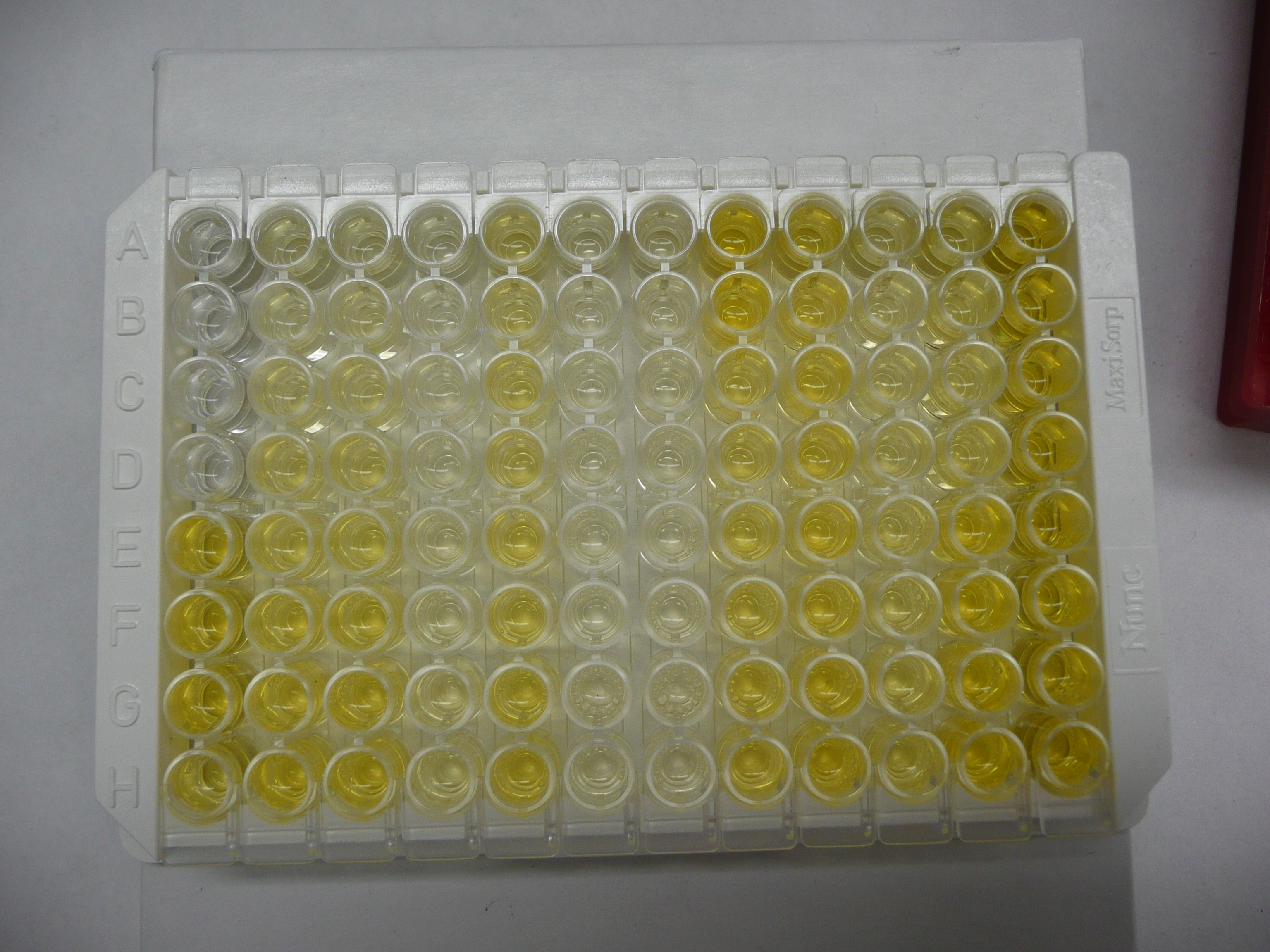
Critiques of my explanation for how this kits works are welcome!

Great post, one small comment.
You said the antibodies will bind and unbind until they reach an equilibrium, for any good antibody this is wrong. Theoretically an antibody can unbind after binding, but this will not happen in RT after any reasonable amount of time. To all intents and purposes, once an antibody is bound, it will never unbind in normal conditions.
This is important, because you must not let your sample or the kit hormone bind first and thus get a competitive edge (unless the kit is used just to bind the free left antibodies, this will reduce the dynamic range).
Thanks, Danny! I made an edit in the video to reflect this information.
Cool explanation. One small nitpick: You say that the hormone and the kit hormone will bind to the antibody receptors in the proportion that they’re present in the solution. Isn’t it slightly more accurate to say that the hormones will bind in a proportion which ON AVERAGE is the same as their proportions in the solution?
With some kind of statistical mumbo-jumbo describing the expected deviations from that mean?
Hi, Morris.
You’re right, it’s probably not EXACTLY the proportion and saying on average would be more accurate. To account for this problem most people (myself included) run all of their samples in duplicate or triplicate. If the 2 values for the same sample are very close, and if all duplicate samples on the plate yield similar values, then we can feel pretty good about getting pretty darn close to the average concentration of the hormone in the sample. In my experience, the values are consistent.
Thanks for helping me clarify!
Kelly
Kelly,
Awesome! Thanks for the explanation! Your excitement is contagious.
-Pip
Kelly,
The term your looking for i believe instead of “glowing” is fluorescing.
Thanks, David!
Hello again,
I’ve never done ELISA (which I prefer to use over EIA, just rolls off the tongue better =P), but a quick look at the internet tells me you aren’t using a fluorescent reagent; Ellman’s reagent is a chromogenic reagent instead (yay wikipedia). This means it’s not emitting light, only absorbing specific wavelengths resulting in a particular color. The plate reader is usually a spectrophotometer, which makes sense given that their purpose is to measure absorption at a particular wavelength.
Hi Kelly,
What brand of dry-erase markers were you using? They look awesome!
It’s not really glowing, at least for the assay above. There are a number of different labels that can be used, and you can use the luminescence (glowing) assays, which use luciferase (a firefly enzyme) that will glow; but the above uses antibodies labeled with an enzyme that cleave a substrate into a yellow byproduct (spectrophotometric), you’ll notice that if you leave the assay out for a long time at room temperature it turns completely yellow, the enzyme never stop working and eventually the samples will reach saturation.
Other labels include fluorescent labels, radioactive labels, etc, the advantage to using an enzyme that continually reacts with a substrate is that even minute quantities of sample will produce finely divided gradients of color change given enough time, the disadvantage is that you can consume all the substrate eventually and oversaturate, i.e. platueau, which depending on starting hormone concentrations may lead to the loss of data.
The technical name for this assay is a competitive binding assay, and unlike most assays (ELISAs being the other commonly used one) the signal produced is inversely proportional to the target hormone/chemical.
Great video, wish I’d had it explained by you instead of learning the hard way. good luck in grad school.
On a side note, make sure that the kit you buy has good antibodies, there’s a lot of crap out there, antibodies that cross-react with other hormone/proteins (non-specific binding) in addition to your target, give bands of the wrong size depending on conditions, etc. Antibodies are non- standard, vary by lot, and about half the papers out there have BIG problems with antibodies. Check publications which your specific lot number, it may save you a lot of grief.